We live in a macroscopic world – almost everything around us that forms a part of our everyday lives is perceptible. Children playing frisbees, a person running in a park, an airplane moving in the sky, planets going around in space – every motion is governed by classical mechanics and obeys Newton’s laws of motion and gravity. But what about the world that is hidden from us – the atomic and subatomic world? Welcome to the realm of Quantum Mechanics!

The word ‘quantum’ in Quantum Mechanics refers to a discrete quantity of something. For example, if we think of sand dunes over a larger region that seems to be continuous, then a quantum of the dunes is a single discrete grain of sand. Similarly, think of an ocean and then, a quantum, in this case, refers to the single molecule of water in the ocean. The word ‘mechanics’ refers to the motion of something.

Quantum Mechanics is a set of mathematical principles that explains and governs the behavior and motion of atoms and sub-atomic particles. Quantum Mechanics forms one of the pillars of physics along with the Theory of Relativity, and String Theory attempts to combine both of them into a unified theory.
Why Quantum Mechanics?
It is a known and well-accepted fact that everything including us is composed of molecules that are made of atoms, which in turn, contain nuclei and electrons. As all the motions in our world obey Newton’s laws, when scientists first studied the electrons and nuclei and their behaviors, they tried analyzing their experimental findings in terms of Newton’s laws, but surprisingly, all such attempts failed.
Over such many experiments, it was deduced that the small sub-atomic particles behave in a way that is not at all consistent with the Newtonian equations. Rather, their structures, energies, and all other properties can only be described with a framework governed by an equation known as Schrodinger’s equation.

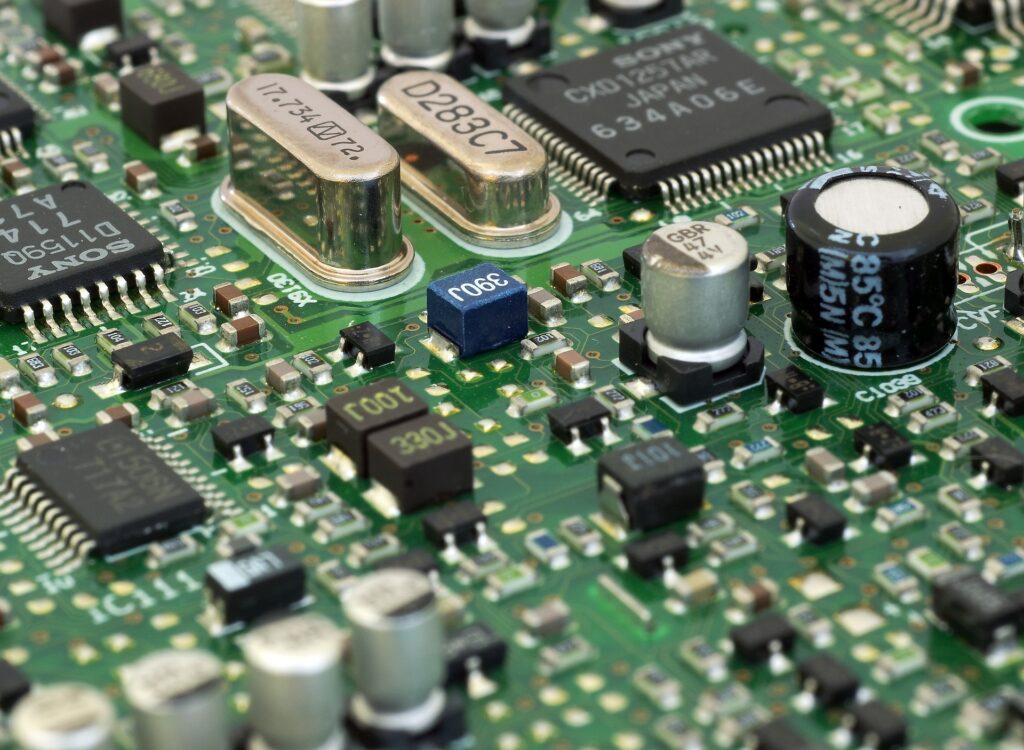
Modern-day computers run on Quantum Mechanics. The motherboard in a computer contains transistors which are made of semiconductors and the foundational working of semiconductors is governed by Quantum Mechanics. In fact, almost any digital device that one can think of, say laptops, cell phones, televisions, all have Quantum Mechanics running its laws in the background. Can we imagine a world without them?
A Brief History of Quantum Mechanics
The origin of Quantum Mechanics is inextricably linked with the wave-particle nature of light. Up till the 19th century, the predominant theory was that light was made of particles (or ‘corpuscular’ as proposed by Newton).
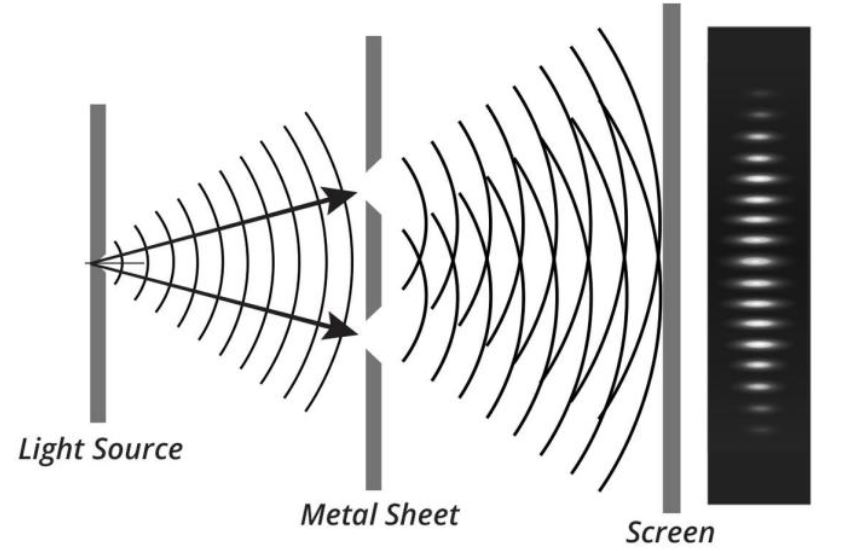
Young’s double-slit experiment in the year 1801 changed the landscape proving that light has wave-like nature too. Similar to ripples or waves on a lake or pond, when two out-of-phase light beams meet, they result in destructive interference, and in the case of in-phase light beams, they reinforce resulting in constructive interference.
Almost a century later in 1905, Einstein also jumped in (he is everywhere!!) and proposed that a light beam is composed of tiny packets of energy and is related to the wavelength of light. He referred them as “das Lichtquant ” or light quanta. Later, these packets of energy came to be known as photons.
Niels Bohr, one of the progenitors of Quantum Mechanics, revolutionized the atomic model in 1913 by proving that the energy of an electron does exist in discrete values, as ‘quanta’, rather than on a continuous scale.

A few years later in 1924, Louis de Broglie found that particles with finite mass such as electrons exhibit wave-like properties with wavelength related to their mass and speed. This completed the hypothesis that if waves can be thought of as particles, then particles can be thought of as waves too.
Louis de Broglie called these waves “Matter Waves”, the equation for which was postulated by Erwin Schrodinger in 1926, and came to be known as Schrodinger’s equation – one of the most important achievements of the 20th century.
Some Fundamental Concepts of Quantum Mechanics
1. Complementary Principle – In the realm of Quantum Mechanics, objects can have properties that can defy intuition such as the particle-like and wave-like nature of an electron. An electron can behave sometimes as a particle and sometimes as a particle, but never can behave like both at the same time.
This is analogous to a coin that has both sides, but when you toss it, it either falls on a head or a tail, never on both. The wave and particle nature of objects can be regarded as the complementary facet of a single reality.
2. Wave function – A wave function is a mathematical function that describes what is going on with the subatomic particle. For the sake of simplicity, we can consider it to resemble a sine function – a composite of crests and troughs.

Just as Newton’s equation, F=ma applies to Newtonian systems, the Schrodinger equation (time-dependent),

applies to quantum systems, where the Greek letter Ѱ represents the system’s three-dimensional wave function.
3. Wave function squared – The Schrodinger equation can very well calculate the wave function of a system, but it does not reveal what a wave function specifically is, and neither anyone knows about it even today.
Max Born, an instrumental person in the development of Quantum Mechanics, proposed that the wave function can be interpreted as a probability amplitude, where the square of the magnitude of the wave function describes the probability of the existence of an electron in a particular location – this changed everything and gave meaning to the wave function.
So, where the wave function is large, we have a larger probability of finding the particle there, and where the wave function is zero, the probability is negligible.
In Quantum Mechanics, a particle, let’s say an electron or a photon, is present in all the locations allowed by the square of the wave function, but at the moment of the detection of the particle’s location, the wave function changes from being in many locations to just the one location where the particle is found – scientists call this moment of detection as the “collapse of the wave function”.

This collapse is hugely debated among physicists and philosophers as it indirectly leads to a claim that an observer plays a critical role and nothing happens in the universe except when an observer makes an observation or a measurement. An extreme example is that of the Many Worlds theory according to which the universe splits into two branches – nearly identical universes whenever a measurement is made.
Deterministic v/s Probabilistic World
As we see, the Schrodinger equation does compute the wave function which is deterministic, but what the wave function describes is probabilistic in nature, and since every subatomic particle is associated with a wave function, the collapse of which leads us to determine its location, this on a large scale describes the things that we see and perceive in our world.

So this idea that nature itself is probabilistic on the most fundamental level is counterintuitive because whatever we see around us is totally deterministic, and that is the beauty of Quantum Mechanics.
“If you think you understand quantum mechanics, you don’t understand quantum mechanics.”
Richard P. Feynman

